)
Welcome to the future of healthcare inventory management.
At RightNow Inventory, our vision is to create an evolved, interconnected healthcare supply chain that ensures every patient receives the right care at the right time. We utilize the latest technologies to innovate inventory management devices and software solutions. These solutions give time back to the healthcare provider to treat patients while protecting the products with layered security that minimizes product waste and maximizes patient safety.
Since 1988
counterfeit free
pharmaceutical distribution
with Guaranteed Channel
Integrity®
Since 2012
innovative forward-deployed inventory management
Solutions
RightNow Secure™
RightNow Secure is a dual temperature zoned, intelligent inventory management system that monitors inventory levels utilizing advanced video imaging technology.
- Controlled Room Temperature
- Refrigerated
- Freezer
RightNow Flex™
RightNow Flex is a space-saving, scalable inventory management solution that monitors inventory levels and temperature readings, ensuring you have the products needed for patient care.
- Ambient
RightNow Ready™
RightNow Ready utilizes pin code security and temperature-control monitoring technologies to provide on-demand access to the pharmaceuticals you need.
- Controlled Room Temperature
- Refrigerated
RightNow Verified™
RightNow Verified is a temperature-controlled inventory management solution leveraging RFID technology to ensure your products are available for patient care.
- Controlled Room Temperature
- Refrigerated
RightNow Cloud™
RightNow Cloud is a virtual, space-saving alternative to an on-premises device that keeps pharmaceutical products stocked for patient care through RightNow Track™.
- Ambient
Solutions
RightNow CloudTM
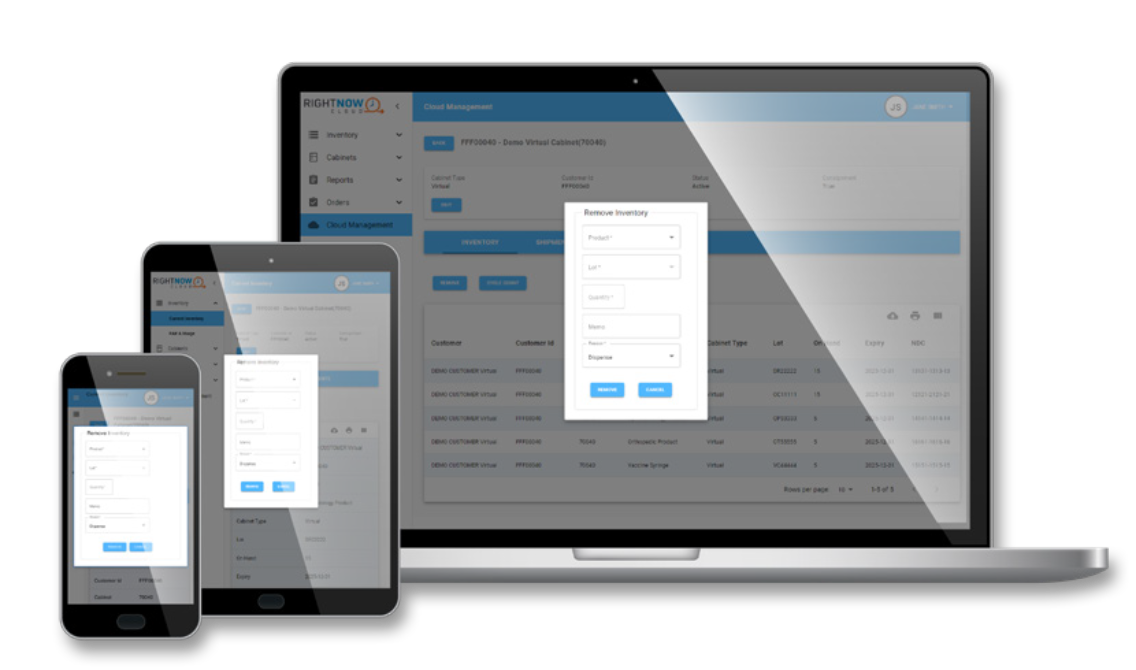
RightNow Cloud is a virtual, space-saving alternative to an on-premises device that keeps pharmaceutical products stocked for patient care through RightNow Track™.
A Suite Of Intelligent Pharmaceutical & Medical
Device Inventory Management Solutions
RightNow Inventory is on a mission to evolve the healthcare
supply chain by developing and deploying inventory management
solutions that confidently maintain inventory at the right
stocking levels, at the right temperatures, and in the right locations.
RightNow.
Immediate Patient Care
Products are available on-site and on-demand when your patients need them.
Efficient Workflows & Visibility
Complete oversight via RightNow Track™ to optimize operational workflow, reduce human error, and eliminate the burden of inventory management.
Patient Focused
We manage product replenishment and alerts, allowing you to focus on patient care.
Greater Savings
Eliminate carrying costs because invoicing only occurs when products are removed from the RightNow solution for patient care.
Providers Prefer RightNow Inventory
In December 2021, we began looking for a vaccine storage solution. From our initial scoping calls, to delivery and setup, to daily usage, to monitoring and troubleshooting, the RightNow Inventory team has been nothing short of phenomenal. They have been professional, responsive, and always willing to help craft solutions that meet the specific needs of our organization.
Sophie Santaella, Chief Operations Officer,
Vinfen Corporation



